
Medical Waste Autoclaves
For Today's Medical Waste Disposal Problems Laws and regulations imposed by local, state and federal agencies dictate that hazardous waste, contaminated with pathogen agents and infectious waste also known as bio-medical or regulated medical waste must be treated and disposed of in a safe and environmentally friendly manner.
 |
Low Volume Medical Waste Autoclave Ideal for laboratories and clinics due to its small footprint and low volume. For more information see Biohazard Sterilizer 5075HSG-BH |
A report on dioxin, issued by the Environmental Protection Agency (EPA), suggests that hospital medical waste incinerators constitute the number one contributor to dioxin in the food chain. Therefore, under the 1990 Clean Air Act Amendments, air emission standards for hospital incinerators handling more than 100 pounds per hour will require full compliance beginning September 1995. This method carried-out by burning the waste, is known to have created an even greater problem due to it's emissions (hazardous flue gas and toxic ash) created during the complex burning process. At the same time different treatment methods were developed, the most proven and internationally accepted one is Steam Sterilization (autoclaving) which became a worldwide trend.
This method transfers hazardous, contaminated waste into a normal non-risk domestic waste in an environmentally friendly, safe and cost effective way.
Canmedic has more then 20 years of experience in the Medical Waste management field and was one of the pioneers in waste treatment systems using autoclaves technology. Canmedic today is one of the leading companies for infectious waste treatment in the world. Its vast experience has enabled Canmedic to offer its customers considerable experience in establishing medical waste management facilities, the autoclaves produced by Canmedic are not only complying with standards (we set the standard).

Door
Each model is available in either single or double door configuration. The doors are automatic horizontal sliding doors and are operated by a pneumatic door mechanism. The door is constructed from AISI 316L grade stainless steel that provides maximum reliability and minimum maintenance. The door closure is secured by means of a high grade silicon profile that is forced against the door by compressed air.
Medical Waste Autoclave Sterilizer
Process
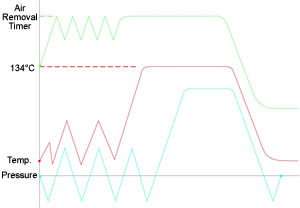
The medical waste sterilizers operate with the technology of a high vacuum steam environment; with steam as the sterilization agent, operating at a pressure of up to 33PSI (2.3 bars) and a temperature of up to 279°F (137°C). Due to the unique design and special construction of the autoclave, together with the regulations requiring a minimum sterilization of 30 minutes, the sterilization cycle is relatively short, the entire cycle is no more than about 50 minutes.
Construction
- Pressure Vessel and Door
Constructed of heavy-duty 316L-grade stainless steel, both single and double door models are available. - Jacket
Constructed of heavy-duty 304L grade stainless steel. - Piping and fittings
Stainless steel and brass. The valves are ball piston type, self cleaning and pneumatically operated. - Mounting
Can be supplied in one of the following mounting alternatives: Built-in, wall mounted, Free-standing and Pit-mounted (floor loading). - Loading
The chamber is supplied with two rails for easy loading. A variety of stainless steel loading equipment is available for your selection.
Doors
The sterilizers can be supplied with one or two doors. The door/s can be fully automatic vertically sliding, horizontally sliding, or hinged with automatic locking. All door types are equipped with special safety device features that prevent the doors from opening while in operation.
Automatic Control Unit
The Medical Waste (BH) sterilizer control system is based on "state-of-the-art" microcomputer technology, guaranteeing high reliability and safe operation. Intervention is unnecessary after selecting the parameters and pressing the start button. The main cycle phases and the machine's actual parameters are displayed on the LCD panel during the cycle progress. The main physical parameters of the process, i.e.temperature, pressure, time and jacket pressure are controlled and displayed. The control system is easy to use and programmable.Calibration of the system can be per formed digitally from the control panel or through a computer communication port via a PC.
Documentation
For a clear and concise documentation of cycle process, the control unit is provided with a 24 character per line printer, which is connected to the microprocessor. In case of an uncompleted cycle, the printout will indicate the cycle failure and the cause of the failure. An additional graphic printer is available,RS485/RS232 PC and Advanced PC Windows-based software for monitoring, logging and control is also available. A circular or trace strip chart temperature and pressure recorder can be supplied on request.
Loading Cart and Carriage
Canmedic offers a complete product range of high-grade Stainless steel (316L) Loading Equipment.
Optional:
Highly automated load in and/or unloading for your sterilizers. The loading equipment is ergonomically designed for safe, simple, easy to use, material handling in your facility.
The (BH) Medical Waste system is designed to dispose of all your infectious waste in an efficient, safe, environmentally friendly and cost effective manner. In compliance with the various international regulations, the following important factors were considered in the Medical Waste (BH: Bio Hazard) Sterilization System development:
1. Produce an autoclave that meets the demands for large, but not necessarily heavy, volume contents and develop a cost effective, efficient system for any-size medical/research facility with a long term solution and a secure disposal method.
2. Prior to sterilizing the medical waste, the air and condensate emitting from the autoclave during the first stages of the cycle as well as the air and condense emitted to the drain during and at the end of the cycle must also be sterilized.
3. A system, which is user friendly. With the sterilization criteria met, Canmedic expanded its medical waste program. Along with the autoclave, Canmedic can provide a complete "turn-key"package including Analysis & Concept, Development and Set up, Implementation and Operation or partial service according to individual requirements.
The (BH) Medical Waste sterilizer operates with saturated steam as the sterilization agent; operating at a pressure of up to 33PSI (2.3 bars) and a temperature of up to 279°F(137°C). While more and more critical diseases are evolving Canmedic autoclaves supply the higher level of sterilization they require.
The High vacuum sterilization method substantially shortens the sterilization cycle, achieved by evacuating the air with a vacuum pump, combined with steam pulsing. A 0.2 µm. HEPA Filter is provided to filtrate the air, which equalizes the vessel pressure. The remaining residue, which is typically substantially less then the original volume, is sterile and may be discarded as municipal waste. Canmedic offers a large range of the Medical Waste (BH) system, from standard 250L up to 4,000L volume, as well as varied sterilizer chamber dimensions according to the specific requirements up to 20,000 liter chambers.
Standards and Directives
The sterilizers are manufactured in accordance with A.S.M.E. standards (American Society of Mechanical Engineers), TUV – (German Standards for pressure vessels), The Medical Waste (BH) sterilizer models meet the provisions of the Medical Device Directive, as well as the Pressure Equipment Directive. In addition, the Quality System is certified in compliance with ISO 9002,EN 46002 and ISO 13488. The devices are UL Listed, comply with the FDA (Food and Drug Administration) guidelines and CE marked.
Automated Material Handling Solutions
The high demands for product sterility, and personnel safety, are met by the application of automated material handling systems. Canmedic offers a product range of automated material handling systems, from a simple loading push/pull system, to highly automated solutions. Proven production operation and years of experience have lead to the design of safe, dependable, high throughput, material handling equipment. The loading automation development was designed side by side with our sterilization systems. This close relationship ensures that material handling is not just an after thought, but an essential component in the layout and design of your facility.
